Chemical Symbols in Reaction Equations
Note that we usually use the chemical symbols for elements and compounds in chemical equations. Thus the equation you just saw on the previous page can be written:
C + O2 → CO2
This equation is read "carbon plus oxygen yields carbon dioxide," with C being the symbol for the element carbon, O the symbol for the element oxygen, and CO2 the symbol for the compound carbon dioxide. It is an example of a process called combustion. During combustion, a reactant combines with oxygen in the air to form a product, in this case carbon dioxide gas.
Recall from the Compounds and Bonds section of this course that elements combine to form compounds in set ratios. The subscript “2” after the CO in CO2 indicates that two oxygen atoms will always combine with one carbon atom when carbon dioxide is produced. This has to do with the law of fixed proportions and the fact that elements will always combine in a ratio that ensures the filling of each atom’s valence shells. It looks like this:
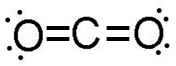
Breaking a compound down into its constituent elements is also an example of a chemical reaction. Can you predict how to write the equation for such a reaction? For example, you can pass electricity through pure water (H2O) to break the water down into its constituent hydrogen and oxygen atoms. The equation looks like this (Don’t worry about where the numbers in the equation come from. You will learn about those later in this unit):
water → hydrogen + oxygen
or
2H2O → H2 + O2