Conservation of Matter
At this point you have learned about the law of conservation of energy and how energy is conserved in chemical reactions. You will now learn about the law of conservation of matter. Like energy, matter is always conserved in a chemical reaction. The law of conservation of matter says that matter cannot be created or destroyed. Therefore, in a chemical reaction, no new atoms are created and none are destroyed. They are only rearranged. You saw this in the previous section when you learned about the types of chemical reactions. In a displacement reaction, for example, one or more elements in a compound switch places with another element. In the diagram below you see a simple reaction of two molecules of hydrogen (H2) reacting with a molecule of oxygen (O2) to form two molecules of water (H2O). Note how the atoms are only rearranged and that nothing is lost or created.
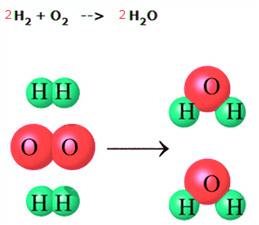
The law of conservation of matter demands that all atoms present in the reactants of a chemical reaction must be accounted for in the products. In other words, there must be the same number of each type of atom on the product side and on the reactant side. Making sure that this rule is obeyed is called balancing a chemical equation for a reaction. Go to the next page to learn more about balancing equations.