Mechanical Advantage
You just learned about the law of conservation of energy. Now, consider an exothermic reaction. For example, methane gas (CH4) is burned to heat many homes in the U. S. The reaction for the combustion of methane looks like this:
CH4 + O2 → CO2 + 2H2O + energy (heat)
Notice that this time we have added energy as one of the products in the reaction. We can diagram this reaction as follows: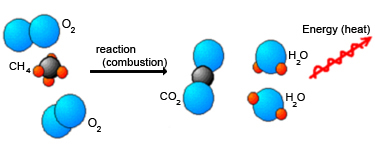
This is an exothermic reaction, and it releases heat to its surroundings, as shown by the red arrow on the right side of the equation. The law of conservation of energy allows us to conclude two important points about this and all other exothermic reactions:
- The energy gained by the surroundings must be equal to the energy lost by the reaction (because the total energy remains constant).
- The energy released to the surroundings was not newly created (because energy cannot be created) but was produced when potential energy stored in chemical bonds was converted to thermal energy.