Nuclear Reactions: Fission
Now let’s take a look at the second form of nuclear change: nuclear fission.
Nuclear fission is a nuclear change in which the nuclei of certain isotopes with a large mass number are split apart into lighter nuclei. Each fission reaction releases two or three neutrons and tremendous amounts of energy. "Fission" means "break apart," or "crack." In nuclear fission, the nucleus of one atom is broken apart, and a few neutrons and a lot of energy is released.
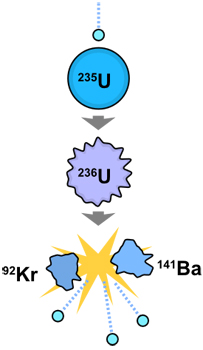
In this diagram, a neutron (the tiny blue dot at top) collides with a uranium 235 atom. This action sets off the initial fission reaction. The uranium 235 absorbs the new neutron and temporarily becomes uranium 236. But uranium 236 is unstable. The uranium 236 nuclei breaks apart, resulting in two fission fragments (Ba141 and Kr92 which are elemental isotopes) and a huge amount of released energy. It also gives off 3 neutrons.
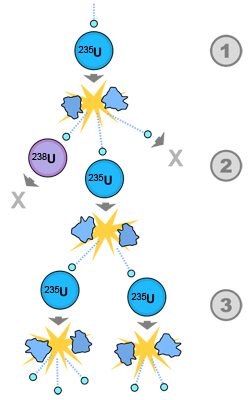
In this image you see how the neutrons released from one fission reaction set off multiple fission reactions. This is called a nuclear chain reaction. Each single neutron triggers a new reaction that produces three more neutrons. This continues exponentially (because each reaction produces more than one neutron), releasing more and more energy each stage along the way.

The atomic bomb is produced by a chain of nuclear fission reactions. You can see the enormity of the energy involved. Radiation, a form of energy, is emitted by the nuclear fission process. It can be powerful enough to damage, sometimes severely, human body tissues. This is why people often speak of nuclear power with fear.