Nuclear Reactions: Radioactive Decay
The final form of nuclear change is natural radioactive decay. Radioactive decay takes place when an isotope spontaneously emits particles, high-energy radiation, or both at a fixed rate. Radiation is just a form of energy that travels from a source. There are several types of radioactive decay, depending on what is emitted from the parent isotope. The diagram shows you a sketch of radioactive decay and four common types: alpha decay, beta decay, gamma decay, and neutron emission. In alpha decay, two protons and two neutrons, which together make an alpha particle, are emitted from the nucleus. In beta decay, an electron is emitted, and in gamma decay, high energy radiation called a gamma ray is emitted by the nucleus.
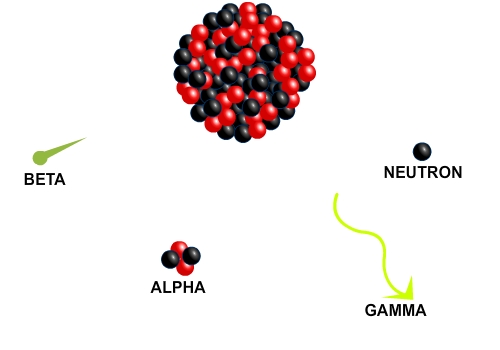
An unstable isotope that decays in one of these ways is called a radioisotope. Any radioisotope decays at a characteristic rate. This rate of decay is expressed in terms of half-life, the time needed for one-half of the nuclei in a given quantity of a radioisotope to decay. An isotope’s half life cannot be changed. Half-lives range from fractions of a second to millions of years, depending on the element involved.
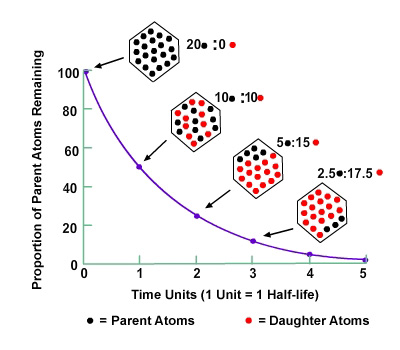
Note here how the amount of the original radioisotope decreases with each successive half-life in time. The diagram shows the atoms of an original material in red dots. It decays at a set rate to what is called the daughter material, represented by black dots. After the elapse of one half-life (one unit in time) there is 50% of the original material left. After the elapse of two half-lives, there is only 25% of the original left, and so forth.